COMPLETE, COST-EFFECTIVE SUPPORT FOR YOUR CLINICAL TRIAL
Project and Supply Chain Management is a field that combines the strategic alignment of project management with the operational mechanics of supply chain management. It focuses on the integration of manufacturing and service operations, logistics, purchasing, and distribution to cultivate value-creating supply chain networks.
Project management involves developing new projects and coordinating procurement and project delivery systems. It’s about managing the flow of work to achieve specific goals within a set timeframe and budget.
Supply chain management (SCM), on the other hand, is the discipline that manages the flow of goods, services, and information from raw material suppliers through factories and warehouses to the end customer. It includes planning, implementing, and controlling the operations of the supply chain as efficiently as possible. SCM is crucial for businesses as it can be a significant competitive advantage, affecting other key business areas such as operations management, inventory control, and quality management.
Together, these disciplines ensure that an organization can effectively manage its resources, deliver products and services to customers, and maintain a competitive edge in the market.
Our project & supply management services are designed to provide comprehensive support for the entire lifecycle of your clinical trial. Our approach is centered around a demand-led supply model that not only reduces waste and costs but also enhances the efficiency and reliability of the clinical supply chain.

Overview
Enhanced Study Design for Cost-Effectiveness: Efficient study design is pivotal for cost containment in clinical trials. By focusing on the core objectives and reducing the complexity of protocols, studies can be both cost-effective and robust. Employing adaptive trial designs allows for flexibility and responsiveness to data as it emerges, potentially reducing the number of participants and resources required. Advanced planning and simulation tools can forecast patient enrollment and study drug needs, ensuring that resources are allocated efficiently and effectively.
Demand-Led Supply for Clinical Trials: A demand-led supply chain is a patient-centric approach that aligns the distribution of investigational medicines with actual patient recruitment and retention rates. This method reduces the need for large inventories and minimizes the risk of drug wastage. By leveraging real-time data and analytics, clinical trials can dynamically adjust supply levels, ensuring that the right amount of medication reaches the right site at the right time. This approach is particularly beneficial for trials involving expensive biologics or therapies for rare diseases, where precision in supply chain management is crucial.
Flexibility and Cost Savings in Supply Chain Management: Decoupling secondary packaging from the primary supply chain introduces significant flexibility and cost savings. This model allows for just-in-time packaging and labeling, which aligns closely with patient enrollment and site activation timelines. It reduces the financial burden of overproduction and storage, and it can adapt quickly to changes in trial protocols or regulatory requirements. A flexible supply chain is not only more efficient but also more resilient to disruptions, ensuring that clinical trials can proceed smoothly even under challenging circumstances.
Innovative Approaches to Clinical Supply Chain Optimization: Innovative strategies such as risk-based optimization and data-driven software can significantly reduce drug waste and cut supply costs. By accurately forecasting demand and employing a continuous manufacturing process, clinical trials can maintain a lean inventory, reducing the environmental impact and enhancing sustainability. These strategies also contribute to a more patient-centric trial experience, as they ensure timely delivery of medications to participants, thereby improving adherence and retention.
Capabilities
Global Supply Chain Project Lifecycle Management
The lifecycle management of a global supply chain project encompasses a comprehensive approach that begins with the manufacturing process and extends through to the final accountability and destruction of products. This process is intricate and multifaceted, involving various stages that require meticulous planning, execution, and monitoring to ensure efficiency and compliance with regulatory standards.
Manufacturing Phase The manufacturing phase is the genesis of the supply chain lifecycle. It involves the production of goods, which must be carried out in accordance with predetermined quality standards and specifications. Effective lifecycle management during this phase includes:
- Design and Development: Establishing product specifications, quality requirements, and identifying necessary resources.
- Production Planning: Scheduling and controlling the production process to meet demand while optimizing resource utilization.
- Quality Assurance: Implementing quality control measures to ensure products meet the required standards.
Distribution and Logistics Once the manufacturing phase is complete, the focus shifts to the distribution and logistics, which involves:
- Transportation Management: Coordinating the movement of goods from the manufacturing site to various distribution centers and retailers.
- Warehouse Management: Storing and managing inventory in a way that maximizes space utilization and minimizes handling costs.
- Order Fulfillment: Processing orders, picking, packing, and shipping products to customers.
End-of-Life Management The final stage of the supply chain project lifecycle is end-of-life management, which includes:
- Accountability: Ensuring all products are accounted for throughout the supply chain to prevent loss and theft.
- Disposal and Destruction: Safely disposing of or destroying products that are no longer usable or have reached their expiry date, in compliance with environmental regulations and company policies.
Supply Chain Planning and Optimization Services Supply chain planning and optimization services are crucial for forecasting future demands and aligning them with supply capabilities. These services include:
- Demand Forecasting: Predicting future customer demand using historical data, market trends, and statistical models.
- Supply Planning: Determining the optimal production and procurement schedules to meet forecasted demand.
- Inventory Optimization: Balancing inventory levels to minimize costs while ensuring product availability.
Coordination and Collaboration with Clinical, Medical, and Third-Party Teams Effective supply chain management in the clinical and medical fields requires close coordination and collaboration with various stakeholders. This involves:
- Clinical Supplies Plan Development: Working with clinical teams to forecast and plan for the necessary supplies throughout the clinical trial phases.
- Monitoring and Adjustments: Continuously monitoring supply levels and making adjustments as needed based on actual consumption and changes in clinical trial protocols.
Integration with IRT Systems Interactive Response Technology (IRT) systems, including Interactive Voice Response (IVR) and Interactive Web Response (IWR), play a pivotal role in clinical supply chain management. Integration with these systems involves:
- Co-Development of Requirements: Collaborating with IRT providers to develop system requirements that align with clinical trial needs.
- Specifications and Monitoring: Establishing specifications for the IRT system and monitoring its performance to ensure it meets the clinical trial’s logistical demands.
Secure Document Sharing and Real-Time Shipment Tracking Maintaining the integrity and security of the supply chain.
Demand Led Supply
Demand-led supply (DLS) is a strategic approach in clinical trial logistics that focuses on increasing efficiency and flexibility in the supply chain. This model is particularly relevant in the context of modern clinical trials, which have become increasingly complex due to the need for targeting smaller patient populations, the high cost of therapies, and the global nature of many studies. Here’s an in-depth look at the efficiencies possible with demand-led supply:
Shorter Timeframe for Clinical Sites to Receive Patient Kits Under the DLS model, the primary packaging of the bulk drug is performed centrally, and the drugs are then shipped to regional facilities located closer to the trial sites. This proximity allows for a much shorter delivery time from the moment a request is made, enabling clinical sites to receive patient kits in days rather than weeks.
Reduced Clinical Waste Traditional clinical trial supply models often require a buffer stock of around 200% to alleviate any potential uneven demand, most of which goes unused. DLS, on the other hand, holds decentralized stock that is not committed until a patient’s need is established, significantly reducing clinical waste to less than 20%.
Drug Pooling Across Protocols DLS facilitates the efficient use of study drugs, including comparators, by allowing for the pooling of supplies across different protocols. This is particularly beneficial when there are multiple programs of work that use the same primary pack bright stock.
Elimination of Booklet Labels The traditional supply chain model involves adding booklet labels to drugs during primary and secondary packaging, which are then stored centrally. DLS uncouples primary and secondary packaging, eliminating the need for booklet labels and thus streamlining the process.
Flexibility to Add/Remove Countries Easily With DLS, the supply chain becomes more adaptable to changes, such as the addition or removal of countries in a clinical trial. This flexibility is due to the decentralized nature of the supply, which can be adjusted more easily to meet the evolving needs of the trial.
Improving Supply Chain Predictability DLS improves supply chain predictability by reducing the risk of supply disruptions and stockouts. This is achieved through a more patient-centric approach that aligns the supply with actual patient recruitment and treatment schedules.
Enhancing Patient Centricity By focusing on the needs of patients and clinical sites, DLS ensures that investigational medicines are delivered on time and in the right quantities, enhancing the overall patient experience and potentially improving adherence to the trial protocol.
24/7/365 Case Manager
24/7/365 Support: Our dedicated case manager provides round-the-clock support, orchestrating centralized inventory management and order aggregation at a global level.
Reduced Timelines
The Crucial Role of Project and Program Management
The pharmaceutical industry is a beacon of hope, constantly pushing the boundaries of science to bring forth life-saving medications. At the heart of this industry’s success is the seamless integration of project and program management with the technical expertise of Chemistry, Manufacturing, and Controls (CMC) teams. This collaboration is not merely a convenience; it is a strategic imperative that ensures the swift and safe delivery of new drugs from conception to the hands of patients.
Strategic Integration for Accelerated Development
Our project and program management experts are the architects of a strategic framework that aligns with the rigorous regulatory landscape and the dynamic nature of pharmaceutical development. By engaging with our CMC team at the inception of the clinical development phase, we lay a robust foundation for scalability and manufacturability. This early-stage collaboration is crucial for anticipating and circumventing the bottlenecks that typically impede the drug development lifecycle.
Risk Management: A Proactive Approach
The journey from a promising compound in clinical trials to a fully-fledged commercial product is fraught with risk. These risks range from scientific and technical challenges to regulatory hurdles and market dynamics. Our proactive risk management approach involves a thorough analysis of the entire development process, identifying potential issues and implementing contingency plans. This foresight is pivotal in maintaining the integrity and momentum of the project.
Navigating Regulatory Complexities
Regulatory compliance is a non-negotiable aspect of pharmaceutical development. Our project and program management teams work in tandem with regulatory affairs specialists to navigate this complex environment. Together, they ensure that every stage of development not only meets but exceeds the stringent standards set by health authorities worldwide.
Scaling Up: From Lab Bench to Global Markets
Scaling up production from clinical to commercial quantities is an intricate process that demands precision and foresight. Our teams meticulously plan every aspect of scale-up, from sourcing raw materials to optimizing manufacturing processes. This planning is critical to ensure that the final product retains its efficacy, safety, and quality on a larger scale.
Ensuring Supply Chain Integrity
A robust supply chain is the lifeline of commercial drug manufacturing. Our integrated approach encompasses supply chain management, ensuring that each link in the chain—from raw material suppliers to distribution networks—is secure, reliable, and compliant with global standards.
The Final Mile: Commercial Launch and Beyond
The commercial launch of a new drug is the culmination of years of dedicated effort. Our project and program management teams work relentlessly to ensure that this final phase is executed flawlessly. Post-launch, we continue to support the product through lifecycle management, monitoring market performance, and implementing enhancements as needed.
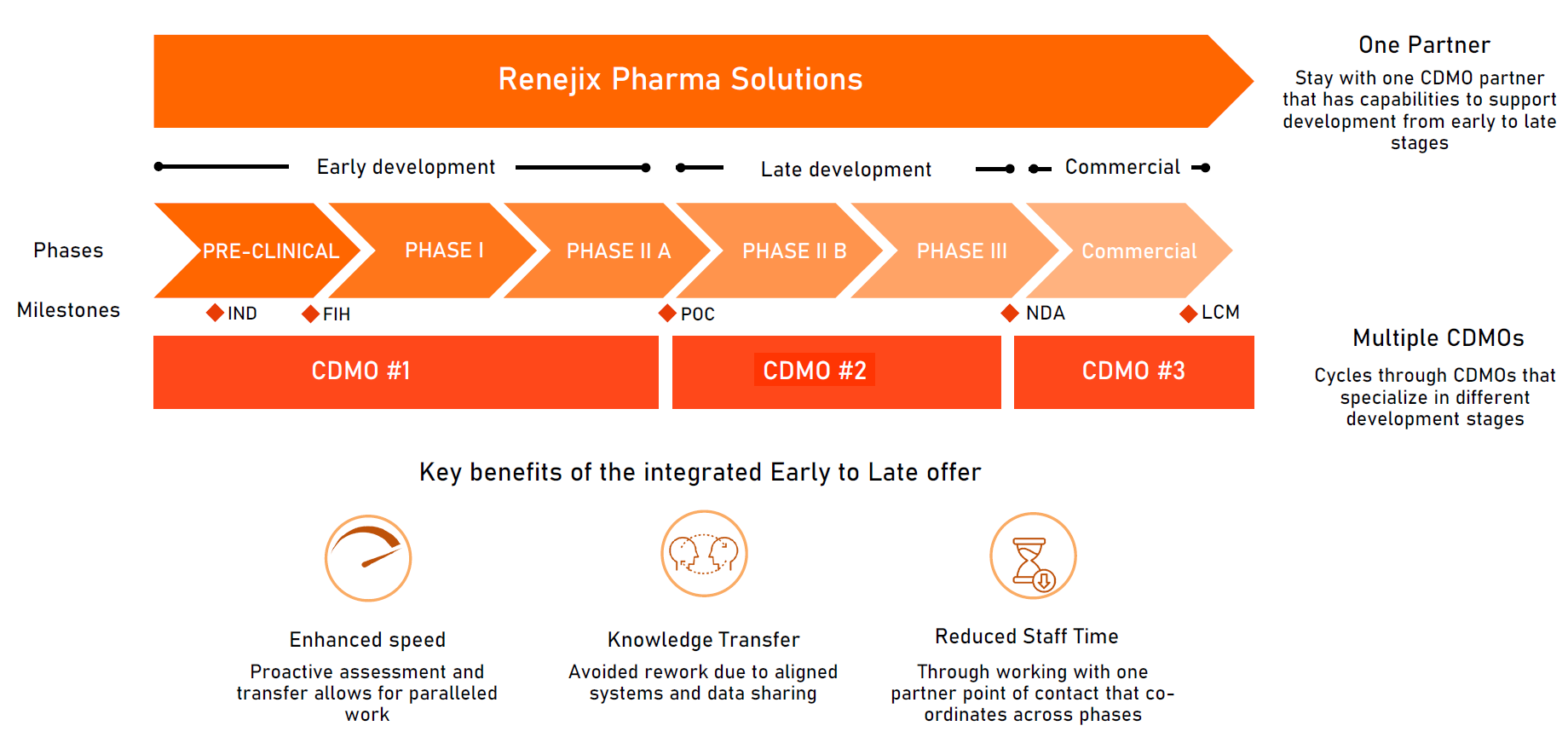
Eliminate the need to cycle through providers, save time with a partner that has capabilities to support development from early to late states
Related Services


Related Content
FAQs
Here are some frequently asked questions about Project & Supply Management
Clinical Trials Project & Supply Management encompasses the planning, coordination, and execution of all aspects related to the supply of investigational medicinal products (IMPs) and other materials necessary for clinical trials. This includes manufacturing, packaging, labeling, storage, distribution, and the management of returns and destruction. The goal is to ensure timely delivery of trial supplies, adherence to regulatory requirements, and alignment with trial timelines and budgets.
We ensure timely delivery of supplies to global trial sites through meticulous planning, robust supply chain logistics, and real-time monitoring. Our team assesses the specific requirements of each trial site, including import/export regulations and local storage capabilities. We leverage a network of certified couriers and use advanced tracking systems to monitor shipments, address any transit issues promptly, and ensure that supplies arrive as scheduled.
To manage supply chain risks in clinical trials, we employ several strategies, including:
- Diversification of suppliers to mitigate the risk of shortages.
- Strategic stockpiling and safety stock management to buffer against supply disruptions.
- Advanced planning and forecasting to anticipate demand and adjust production schedules.
- Continuous monitoring of the supply chain for potential risks and implementing contingency plans as needed.
For multicenter trials, we implement a centralized supply management strategy to ensure consistency and efficiency. This involves coordinating with all trial sites to understand their specific requirements, regulatory considerations, and enrollment timelines. We utilize centralized warehousing and distribution models to streamline logistics and maintain control over the inventory, ensuring that each site receives the necessary supplies according to the trial schedule.
Yes, we can support adaptive trial designs with flexible supply management solutions. Our systems and processes are designed to accommodate changes in trial protocols, including adjustments to dosage, patient cohorts, or study endpoints. We work closely with sponsors to anticipate potential adjustments and implement scalable solutions, such as on-demand packaging and labeling, to quickly adapt to the evolving needs of the trial.
We ensure compliance with regulatory requirements for trial supplies through rigorous quality assurance processes, adherence to Good Manufacturing Practices (GMP), and regular audits. Our regulatory affairs team stays updated on global regulations and works closely with sponsors and trial sites to ensure that all documentation, labeling, and shipping procedures meet the specific requirements of each jurisdiction where the trial is conducted.
For the secure storage and handling of trial supplies, we employ state-of-the-art facilities with controlled temperature and humidity conditions, as required. Our warehouses are equipped with security systems, access controls, and 24/7 monitoring to protect against unauthorized access and environmental hazards. We also implement strict handling procedures, including segregation of products and thorough training for personnel, to maintain the integrity of trial supplies.
We manage the labeling and packaging of clinical trial supplies by offering customized solutions that meet the specific requirements of each trial, including multi-language labeling, blinding, and patient-centric designs. Our facilities are equipped to handle a range of packaging formats, and our quality control processes ensure that all materials meet the highest standards for accuracy, readability, and compliance with regulatory guidelines.
Yes, we provide real-time visibility into the supply chain for sponsors through our advanced inventory and shipment tracking systems. Sponsors can access up-to-date information on stock levels, shipment status, and site deliveries through a secure online portal. This transparency allows for proactive management of supplies, informed decision-making, and timely responses to any challenges that may arise.
At the end of a study, we conduct a thorough reconciliation of unused supplies to account for all materials distributed and returned. This process ensures compliance with regulatory requirements and supports the accurate reporting of trial data. Unused supplies are then disposed of in accordance with environmental regulations and documented procedures, including secure destruction of sensitive materials. We provide comprehensive documentation for the reconciliation and destruction process to sponsors for their records.