Renejix specializes in formulating oral films for oral and mucosal delivery. They offer several benefits:
- Oral films excel in rapid disintegration and fast drug release realize easy dose without water nor effort.
- Various masking methods suppress bitterness and convergence enhances good mouth feeling during administration.
- The thin film dissolves quickly in the mouth and is absorbed into the body. Since it is orally taken without water, it is suitable for elderly patients with swallowing difficulties and bedridden patients.

Overview
- Rapid Therapeutic Action: Oral films are renowned for their almost instantaneous dissolution, which ensures swift medication delivery. This rapid action is particularly crucial for conditions that require immediate therapeutic intervention.
- Ease of Administration: Oral films are ingeniously designed for discreet administration without the need for water, making them exceptionally convenient for patients with dysphagia or those who are on-the-go.
- Precision in Dosing: Oral films are engineered with precision to deliver exact dosages. This meticulous engineering maintains consistent therapeutic levels and minimizes the potential for side effects.
- Bioavailability Optimization: The direct dissolution of oral films in the oral cavity optimizes bioavailability. This efficient delivery method effectively bypasses hepatic metabolism, enhancing the drug’s absorption.
- Formulation Flexibility: Oral films exhibit remarkable formulation flexibility, capable of incorporating a diverse spectrum of small molecule drugs to cater to various therapeutic needs.
- Stability and Longevity: The stability and longevity of oral films are significant, as they can prolong the active ingredient’s stability, offering an extended shelf life compared to conventional dosage forms.
- Scalable and Innovative Manufacturing: Oral films are produced using state-of-the-art, innovative manufacturing processes that are fully scalable, efficiently meeting commercial demands.
- Patient-Centric Design & Patient Compliance: Oral films are developed with a focus on patient comfort and convenience, ensuring easy integration into daily routines without disrupting lifestyle choices. The user-friendly nature of oral films, coupled with the potential for flavor enhancement, significantly improves patient adherence to medication regimens.
- Technological Advancements: Continuous technological advancements in oral film formulations are driving the development of more effective and patient-friendly drug delivery systems.
- Innovation in Flavoring: We are innovating in the realm of flavoring to make oral films more palatable, thereby enhancing the overall patient experience and adherence.
Tailored Formulation Development
- Customized Drug Release Profiles: Renejix’s expertise in oral films formulation development includes creating customized drug release profiles. Whether the therapeutic need calls for immediate or controlled release, our oral films are meticulously designed to meet the precise elution profile of the drug.
- Combination of Multiple APIs: Our advanced systems are expertly formulated to incorporate multiple active pharmaceutical ingredients (APIs) into a single oral film. This allows for a more comprehensive treatment approach, addressing multiple symptoms or conditions effectively.
- Nanotechnology Integration: Renejix can integrate nanotechnology into the development of oral films to enhance drug solubility and bioavailability. This innovative approach allows for lower dosages and reduced side effects, improving patient outcomes.
- Molecular Characterization: State-of-the-art technology is employed for the molecular characterization of our oral films, ensuring the integrity and consistency of the product from batch to batch.
- Advanced Production Methods:
- Solvent Casting: Renejix utilizes solvent casting, a prevalent technique for creating uniform and consistent oral films, which is crucial for dose accuracy and patient safety.
- Hot-Melt Extrusion: For oral films that require a higher degree of physical stability or contain heat-sensitive components, Renejix employs hot-melt extrusion, ensuring the durability and efficacy of the films.
- Flavoring and Sensory Optimization: We focus on flavoring and sensory optimization to make our oral films more palatable, enhancing the overall patient experience and encouraging consistent use.
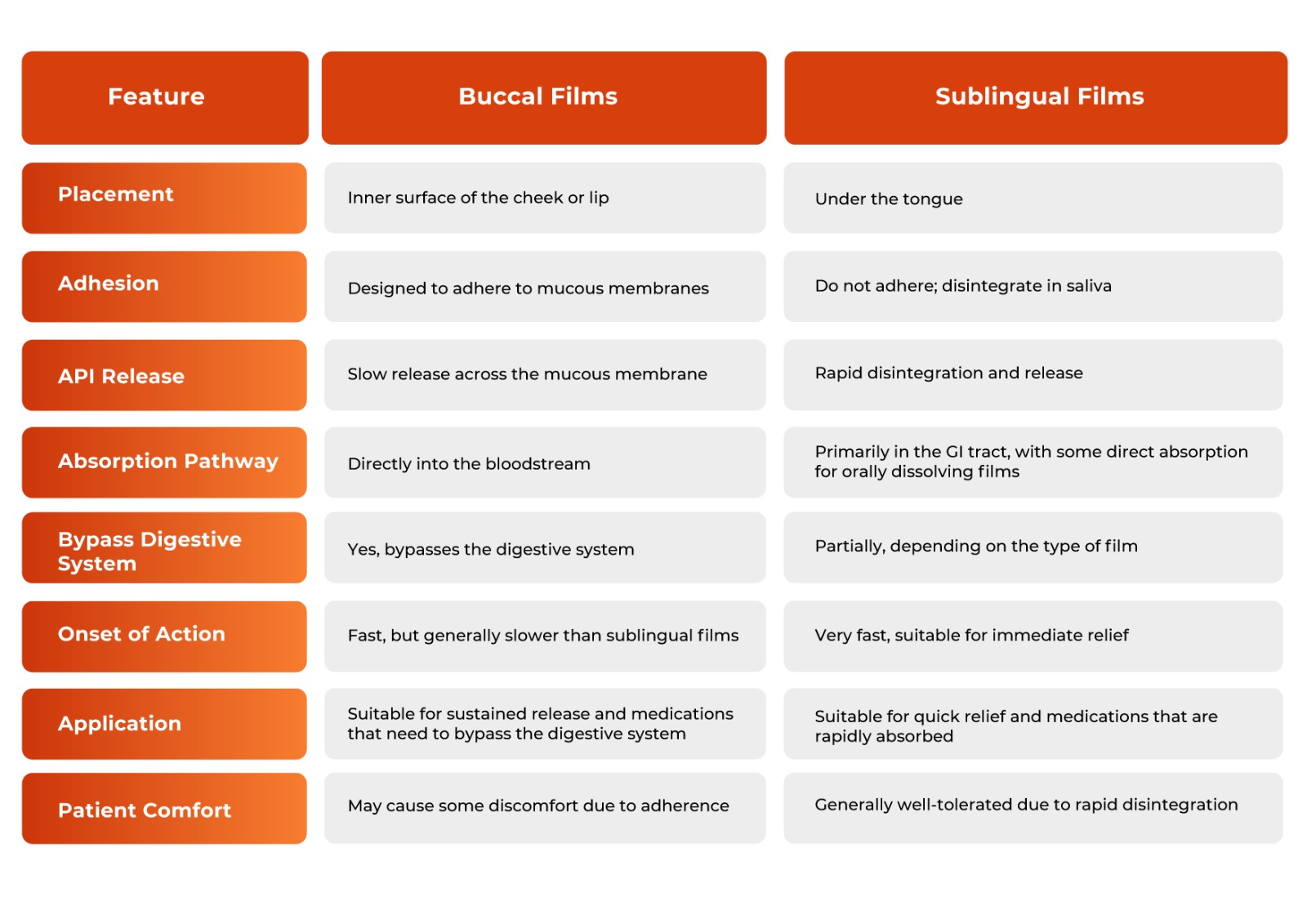
Buccal Films and Sublingual Films Comparison
Advanced Manufacturing Techniques
- Cutting-Edge Manufacturing: We employ cutting-edge manufacturing techniques to ensure the highest quality of oral films. This begins with selecting a suitable polymer blend or binder, crucial for the film’s strength, stability, and mucoadhesiveness.
- Precision Coating and Drying: Our Oral Films undergo precision coating and drying processes to ensure uniformity in the drug’s potency and the film’s thickness, tailored to specific therapeutic needs.
- Encapsulation Technologies: We utilize advanced encapsulation technologies in our oral films to protect the drug from environmental factors and precisely control its release profile.
- Analytical Testing: Our Oral Films are subject to rigorous analytical testing, employing advanced methods to assess purity, potency, and uniformity, ensuring the highest quality standards.
- Solubility and Bioavailability Enhancement: To increase the bioavailability of poorly soluble APIs, we incorporate techniques like amorphous solid dispersions and nano- and microparticle technologies into our oral films.
- Uniformity: Our manufacturing process guarantees uniform distribution of the API within the oral films, ensuring consistent dosing with each administration.
- Scalability: Our oral films manufacturing facilities are designed for high throughput, capable of accommodating large-scale production without compromising on quality.
- Material Efficiency: We optimize our oral films manufacturing process to reduce pressure loading on drug substances, minimize the loss of raw materials, and expand possibilities in drug formulation development.
- Process Optimization: Our process minimizes the amount of solvent and heat transferred to drug substances, reducing pressure loading and raw material loss between processes.
- Product Specifications: Our oral films are typically a few square centimeters in surface area and range from 50 to 150 μm in thickness, designed for rapid dissolution and ease of use.
- Environmental Considerations: We are committed to environmentally responsible manufacturing practices for our Oral Films, aiming to reduce our ecological footprint.
- Regulatory Expertise: Our team has extensive expertise in navigating the complex regulatory landscape, ensuring that our Oral Films meet all necessary compliance standards.
- Quality Assurance: We implement stringent quality assurance protocols for our Oral Films, ensuring that each product meets our high-quality benchmarks.
Advantages
- Microbial Resistance: The physical structure of oral films, coupled with their low moisture content, creates an inhospitable environment for microbial growth, enhancing the product’s safety and longevity.
- Enhanced Stability and Shelf Life: Oral films offer greater stability and shelf life compared to traditional dosage forms. Their robust structure and moisture-resistant properties contribute to their extended usability.
- Resistance to Physical Degradation: Unlike traditional solid dosage forms, oral Films are thin, flexible strips of polymer that are not friable. This allows them to resist the kinds of physical degradation that would damage normal tablets and capsules.
- Packaging-Induced Stability: The packaging of oral films contributes to their stability. Each film strip can be individually wrapped in flat, sealed packages that are practically devoid of air, protecting them from atmospheric moisture and oxygen until administration.
- Discreet and Portable: Oral Films are a discrete and easily portable dosage form. Their compact size and individual packaging make them ideal for patients who are traveling or on-the-go.
- Rapid-Release Profile: Oral films possess the same rapid-release characteristics as a typical liquid suspension, providing quick therapeutic action which is essential for conditions requiring prompt relief.
- Patient Compliance: The convenience and ease of use associated with oral films significantly improve patient compliance, especially for those who have difficulty swallowing pills or following complex medication schedules.
- Taste Masking: Oral films can be formulated with taste-masking agents, making medication palatable and improving the overall experience for the patient.
- Innovative Formulation: Renejix’s oral films are developed using innovative formulation techniques that allow for the integration of various therapeutic agents, catering to a wide range of medical conditions.
- Child Safety: Our oral films are designed with child safety in mind, featuring packaging that is difficult for children to open, thereby reducing the risk of accidental ingestion.
- Customization for Special Populations: We offer customization of oral films for special populations, such as pediatric or geriatric patients, ensuring that the medication meets their specific needs and preferences.
Packaging
- Moisture Control: To safeguard the integrity of oral films, our packaging materials are meticulously chosen for their superior moisture barrier properties. This ensures that the films are well-protected from degradation due to environmental moisture.
- UV Protection: Our Oral Films are shielded from harmful UV rays with specialized packaging. This preserves the stability of light-sensitive active pharmaceutical ingredients (APIs), maintaining the efficacy of the medication.
- Tamper-Evident Features: The packaging for our oral films includes tamper-evident features. These are designed to show clear evidence of tampering, thus ensuring the safety and security of the patient.
- Advanced Desiccants: We incorporate advanced desiccants into our oral films packaging to actively control humidity levels, further protecting the films from moisture-induced degradation.
- Child-Resistant Design: Our oral films packaging are designed to be child-resistant, providing an additional layer of safety by preventing accidental access by children.
- Portability and Convenience: The design of our oral films packaging emphasizes portability and convenience, making it easy for patients to carry their medication without the risk of damage or exposure.
- Branding and Information: The packaging of our oral films is not only functional but also informative, with clear branding and essential information about the medication for patient education.
Related Services





FAQs
Here are some frequently asked questions about Oral Films
Oral suspensions and solutions are two popular liquid dosage forms used for the administration of medications. An oral solution is a clear, homogeneous liquid containing one or more dissolved substances, typically intended for ingestion. Oral suspension, on the other hand, consists of solid particles dispersed throughout a liquid in which they are not soluble, requiring shaking before administration to ensure dose uniformity. Both forms are designed to improve ease of use, enhance patient compliance, and provide an effective means of dose adjustment.
A wide range of drugs can be formulated into Oral Films, including pharmaceuticals aimed at treating acute conditions requiring rapid onset of action, chronic medications for improved compliance, and pediatric or geriatric medications where swallowing difficulties are common. Suitable drug candidates include those with appropriate potency, stability, and solubility profiles. However, drugs with very high dose requirements or poor water solubility may present formulation challenges.
Our development process for Oral Films starts with a feasibility assessment, considering the drug's chemical properties, dose, and targeted release profile. We then proceed with formulation development, optimizing the film's composition to ensure drug stability, effective release, and patient acceptability. This includes selecting appropriate polymers, plasticizers, and taste-masking agents. The process is supported by rigorous testing for dissolution, mechanical strength, and shelf life. We utilize Quality by Design (QbD) principles throughout development to ensure robust and scalable formulations.
Our CDMO is equipped with advanced manufacturing technologies for Oral Films, including solvent casting, semi-solid casting, and hot-melt extrusion. We can support small-scale batch production for clinical trials up to large-scale commercial manufacturing. Our facilities are designed to handle various film sizes, thicknesses, and active ingredient loads, with inline quality control measures to ensure consistency and compliance with specifications.
Quality and regulatory compliance are integral to our operations. We manufacture Oral Films according to current Good Manufacturing Practices (cGMP), and our quality control labs are equipped to conduct comprehensive testing, including physical, chemical, and microbiological analyses. We work closely with regulatory authorities to ensure that all products meet the necessary standards and requirements for safety, efficacy, and quality. Our regulatory affairs team provides support for dossier preparation and submission, facilitating product approvals in various markets.
Oral Films offer several advantages, including improved patient compliance due to ease of administration without water, fast dissolution and absorption for quicker onset of action, precise dosing, portability, and the ability to bypass first-pass metabolism for certain drugs, potentially improving bioavailability. They are also ideal for pediatric, geriatric, and neurodiverse populations who may have difficulty swallowing pills.
Yes, Oral Films can be formulated with multiple APIs, allowing for combination therapies that can improve treatment efficacy and patient compliance. The development of multi-API films involves careful consideration of the compatibility and stability of the APIs within the film matrix, as well as ensuring that each API is released in a manner that achieves the desired therapeutic effect.
Taste masking is critical for patient acceptance of Oral Films. We employ several strategies to mask unpleasant tastes, including the use of sweeteners, flavors, and taste-masking agents that encapsulate the API and prevent interaction with taste receptors. We also optimize the film's dissolution rate to minimize the duration of taste exposure. Our formulation experts work closely with clients to achieve a balance between taste masking and drug release profiles.
Our regulatory affairs team offers comprehensive support for the regulatory submission of Oral Film products, including dossier preparation, regulatory strategy development, and interaction with health authorities. We provide all necessary documentation, including quality, non-clinical, and clinical data, to support the application for product approval. Our expertise spans various regulatory frameworks, ensuring efficient navigation of the submission process in different markets.
Pharmaceutical companies interested in Oral Film development or manufacturing can start by contacting us through our website, email, or phone. An initial consultation will be scheduled to discuss the project's scope, objectives, and specific requirements. Following this, we will provide a detailed proposal outlining our services, timelines, and cost estimates. Once the proposal is accepted, a project manager will be assigned to oversee the project from initiation through to completion, ensuring seamless communication and project execution.