INTRODUCTION
The development of new chemical entities (NCEs) has shifted in recent years toward smaller drug developers, creating a more varied market pipeline. Although more money has been put into research and development, and there are more molecules available, the FDA’s approval rate for oral drug candidates has remained largely constant.
In addition to rising development costs, one of the main causes of this declining return on investment is the attrition rate, which is consistently high, especially in the final stages of clinical trials. The difficulties with commercialization, efficacy, clinical safety, and non-clinical toxicology are to blame for this attrition.
The fact that the majority of NCEs struggle with bioavailability issues like insufficient solubility and permeability, which prevent their absorption into patients’ digestive tracts, only serves to exacerbate the problem.
To address this, formulators must employ bioavailability enhancement (BAE) technologies to create effective dosage forms.
However, it is important to avoid various pitfalls that can cause attrition before using BAE technologies on a molecule during dose formulation. These pitfalls include a lack of physicochemical characterization, a lack of integration between early development stages and development of the overall process, and a conduct failure of biopharmaceutics and pharmacokinetics studies.
Overall, formulation technologies that can solve bioavailability problems and reduce attrition risks at various stages of drug development are crucial to the success of the next generation of small-molecule oral medications.
The complexity of drug development can be difficult for smaller pharmaceutical companies to prepare for because they may not have the necessary internal knowledge.
As a result, they frequently need to work with outside partners, which could lead to information gaps between various stages of the development process.
However, making proactive decisions early on can help to lessen these difficulties. Drug manufacturers can improve their chances of completing Phase I trials by utilizing screening contemporary early technologies processes and utilizing the development in the data gathered throughout.
The choice of the active pharmaceutical ingredient (API) and the formulation of the drug are two areas where discontinuity frequently occurs in the early stages of drug development.
These procedures are sequential, beginning with the selection of the salt and polymorphic forms of the chemicals by chemists, followed by formulators who design an appropriate drug delivery method and often employ bioavailability enhancement (BAE) technologies.
DISCONTINUITY IN EARLY DRUG DEVELOPMENT
Unfortunately, these processes can become disjointed in reality, especially for smaller businesses. Early collaboration with a contract development and manufacturing organization (CDMO) that has extensive pre-formulation, analytical, and formulation capabilities can help to address this disconnect. Such cooperation has been shown to produce advantages by lowering costs, saving time, and ensuring easier changes between stages.
When chemists create a salt form, it might not improve solubility enough to get around bioavailability issues brought on by poor solubility alone. Additionally, when choosing a formulation, they frequently do not take the molecule’s performance into account.
Involving formulators early in the process can therefore close the gap and allow for a more integrated strategy.
The scientists involved in the discovery phase are not in charge of administering the molecule to patients. Instead of downstream processes, their main attention is on elements like solubility, purity, and manufacturability. As a result, it is frequently too late to make significant changes once the formulator receives the molecule because it may work in a different company or division than the chemist. Since the salt and polymorphic forms have already been established, changing them later on is difficult and expensive.
Drug companies can use an integrated strategy known as parallel screening to close the gap between API development and formulation.
This strategy entails gathering data all along the molecule discovery process and utilizing it when choosing the dose form.
Formulators can learn early on about an API’s biopharmaceutical constraints by implementing parallel screening. They can then decide which BAE technology is best for converting the molecule into a promising drug candidate. Drug companies can prevent possible delays and repeated efforts in later stages of development by doing this.
EVALUATION OF THE NEW CHEMICAL ENTITY IN STEP 1 (NCE)
Each NCE has a distinct structure that governs its potential to function as an active pharmaceutical ingredient (API). However, creating a molecule with strong activity alone does not ensure the success of a drug.
To identify the biopharmaceutical issues that must be resolved when the NCE is formulated into a workable oral drug, it is critical to conduct thorough pre-formulation characterization studies early on.
The following information was gathered during thorough molecular characterization:
- In biological media, solubility
- Dimensionality and polymorphism
- Hygroscopicity
- Stability in various circumstances (e.g., light, oxygen, temperature)
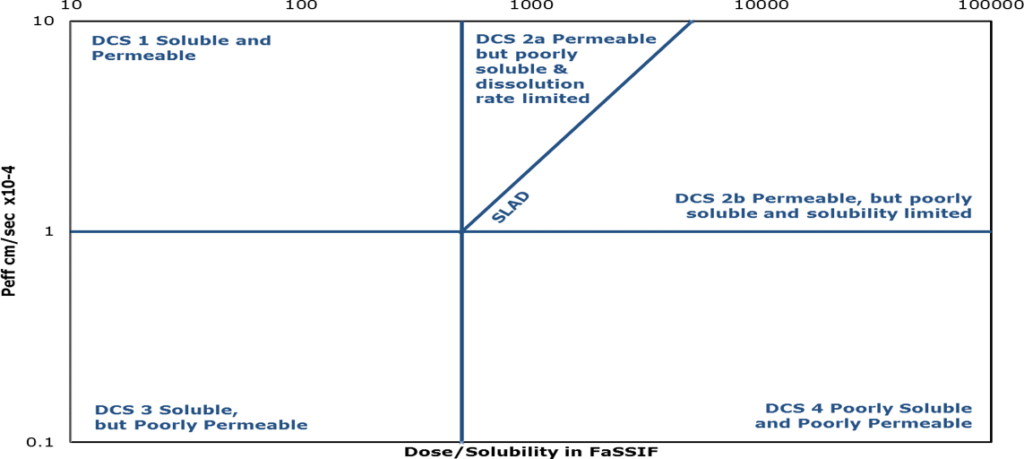
Following thorough characterization, formulators can use a model to match the specifics of the API with the most appropriate delivery method. Utilizing the Developability Classification System (DCS) is a practical strategy.
The FDA’s Biopharmaceutics Classification System (BCS), which has long been employed as a predictive tool for determining the oral bioavailability of an NCE, serves as the foundation for the DCS. The DCS classifies molecules into one of four groups according to their unique solubility and permeability challenges, much like the BCS model does. The DCS, on the other hand, goes a step further and subcategorizes Class II into Class IIa for molecules with limited absorption due to dissolution rate and Class Iib for molecules with limited absorption due to solubility. The DCS Class II category includes the equivalent of 70% of pipeline drug candidates globally.
Formulators can choose the best formulation strategies and technologies required to address the unique biopharmaceutical challenges of the molecule by using the DCS and comprehending the NCE’s classification indicating that they are adequately permeable but poorly soluble.
The position of a molecule on the DCS plot gives guidance (but not necessarily a conclusion) for which formulation technology can successfully address a molecule’s particular bioavailability issues.
Formulators can be directed to the delivery technology that has the best chance of success by using the DCS as a starting point.
Class II: Poor solubility + Good permeability About 70 percent of API candidates fall into this class, and will likely undergo solubility enhancing formulation – depending on if they are Class IIa (dissolution rate limited) and Class IIb (full dose not expected to dissolve before leaving small intestine). | ||
Class III: Good Solubility + Poor PermeabilityMay need to undergo permeation enhancement. Tablets or capsules will likely be achievable. | ||
Class IV: Poor Solubility + Poor PermeabilityThese candidates may be viable if solubility and/or permeability enhancing technologies are used. |
Step 2: Increasing Bioavailability
The next step is to investigate appropriate bioavailability enhancement (BAE) technologies that can efficiently deliver the API into the patient’s digestive system after the distinctive properties of a molecule have been evaluated. There are three widely used techniques for doing this:
LIPID-BASED DRUG DELIVERY SYSTEMS (LBDDS)
For NCEs categorized as DCS Class IIb, which have solubility restrictions, LBDDS is particularly effective. In these circumstances, the NCE cannot completely dissolve before leaving the small intestine. LBDDS involves securing the molecule in solution or suspension by solubilizing it with the aid of lipophilic, hydrophilic, or mixed vehicles. LBDDS is widely used and has been successful in commercializing more than 60 new molecules (NDA). As the API is formulated in solution, LBDDS not only improves bioavailability but also provides superior dose uniformity and scalability for powerful drugs. Early-phase molecules that are formulated in LBDDS frequently result in the creation of durable and adaptable dose forms like softgels, which offer a variety of shell formulations and a large selection of excipient options.
The dispersion and digestion properties of soft gels can be tailored for applications requiring immediate, controlled, or targeted release.
To protect the formulation and the molecule from the acidic environment of the stomach and enable release in the intestine or colon for optimal exposure, enteric-coated capsules or semi-solid fills can be used.
LBDDS has a long history of commercial success and has been the subject of extensive research for solubilization. With its uses constantly growing, it has been crucial in the creation of hormones, omega-3 fatty acids, vitamin D, retinoids, and life-saving medications like protease inhibitors.
Please be aware that LBDDS is one of the three main BAE technologies; the other two will be covered in the steps that follow.
DCS Class III:
LBDDS can be used to address permeability issues for substances classified as DCS Class III substances, such as specific macromolecules
For instance, peptides frequently have good aqueous solubility but poor permeability, which limits their ability to be absorbed in the gastrointestinal (GI) tract.
LBDDS formulations can include permeation enhancers to boost paracellular transport and momentarily relax the tight junctions between enterocytes. This enhances macromolecules’ absorption by allowing them to pass through.
The absorption of macromolecule drugs can be enhanced by combining LBDDS with permeation enhancers Generally Recognized as Safe (GRAS) excipients.
This may make it possible for biotherapies that are typically administered via injection due to poor permeability to be administered orally.
Amorphous Solid Dispersions (ASDs):
Another BAE technology that is gaining popularity is ASDs, especially for molecules with solubility issues that are classified as DCS Class IIb.
In ASDs, the API is changed from its crystalline form to an amorphous form, which by nature has better solubility. The amorphous API, however, is metastable and susceptible to crystallization. Formulators incorporate polymers into the formulation to stop this from happening resulting in a solid dispersion that preserves the API’s amorphous state.
These solid dispersions increase the API’s solubility and rate of dissolution, increasing its bioavailability.
Formulators can achieve desired drug release profiles and stabilize the amorphous form by carefully choosing and optimizing the polymer and excipients.
ASDs provide a flexible method to increase Class Iib molecules’ bioavailability, increasing their potential for oral delivery and addressing the solubility challenges that hinder their efficacy as oral drugs.
Particle Size Engineering:
A popular technique for increasing the oral bioavailability of NCEs with dissolution rate restrictions is particle size engineering. Class IIa, Class IIb-borderline, and Class IV molecules can all be used with this formulation technology. There are two main approaches:
- Milling: This technique works well for generating uniform particle sizes greater than 20 microns. To produce a batch of the molecule that is homogeneous and uniform, the API must be physically altered.
- Micronization: Through high-velocity particle-to-particle impacts, micronization uses air jet micronizers to reduce molecule particle size until the desired size is reached. Since no heat is produced during this high-energy procedure, the API’s thermodynamic stability is maintained. Micronization is especially helpful for keeping the molecule’s solubility and rate of dissolution intact.
Formulators can also choose co-micronization by adding excipients to boost the dissolution rate and possibly increase solubility.
Conclusion
Drug companies should adopt a data-driven and connected approach from molecule selection to dose form selection if they want to leverage solubility-enhancing effectively. Drug developers can evaluate and improve NCEs based on their unique biopharmaceutical characteristics by using parallel screening, avoiding bioavailability-related difficulties in the later stages of the development process. Working together with companies like Hycon, which provide thorough pre-formulation and formulation screening capabilities, offers a quick way to realize the full potential of novel compounds and increases the likelihood that an NCE will be successfully advanced through human trials.






