cGMP vs GMP: What’s the Difference?
Maintaining high standards of quality and safety is essential in pharmaceutical manufacturing. Two fundamental concepts that guide this industry are Good Manufacturing…
Renejix – Small Molecule CDMO (USA)
Maintaining high standards of quality and safety is essential in pharmaceutical manufacturing. Two fundamental concepts that guide this industry are Good Manufacturing…
The debate between biologics and small molecule drugs is pivotal for clinicians, researchers, and patients alike. Each type of medication offers distinct…
Drug formulation is an essential component of pharmaceutical research and development, involving a complex process of combining an active pharmaceutical ingredient (API)…

Clinical trial supply involves the management of materials required to conduct clinical trials, an important aspect of medical research. These materials range…
In drug development, preclinical development acts as a critical bridge between the theoretical design of new compounds and their practical application in…

Oral suspensions and oral solutions are commonly used pharmaceutical formulations for the administration of medications. They provide a convenient and effective way…

Oral Films represent a groundbreaking advancement in pharmaceutical technology, offering a novel approach to drug delivery that combines convenience, efficacy, and patient…
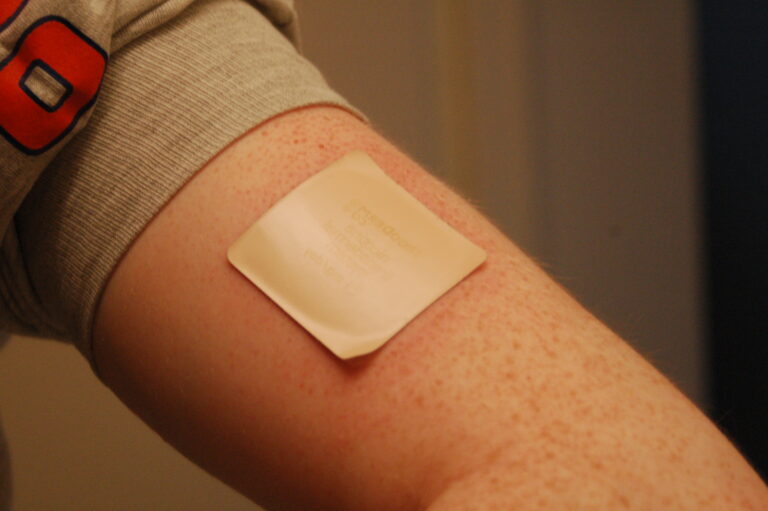
Transdermal patches have become a popular method of drug delivery, offering a convenient and non-invasive way to administer medications. These patches deliver…

Semi solid dosage forms (SSDFs) represent a pivotal category in pharmaceutical formulations, bridging the gap between liquid and solid medications. Characterized by…

The pharmaceutical industry is a cornerstone of modern healthcare, providing innovative solutions to an array of medical challenges. The journey from a…