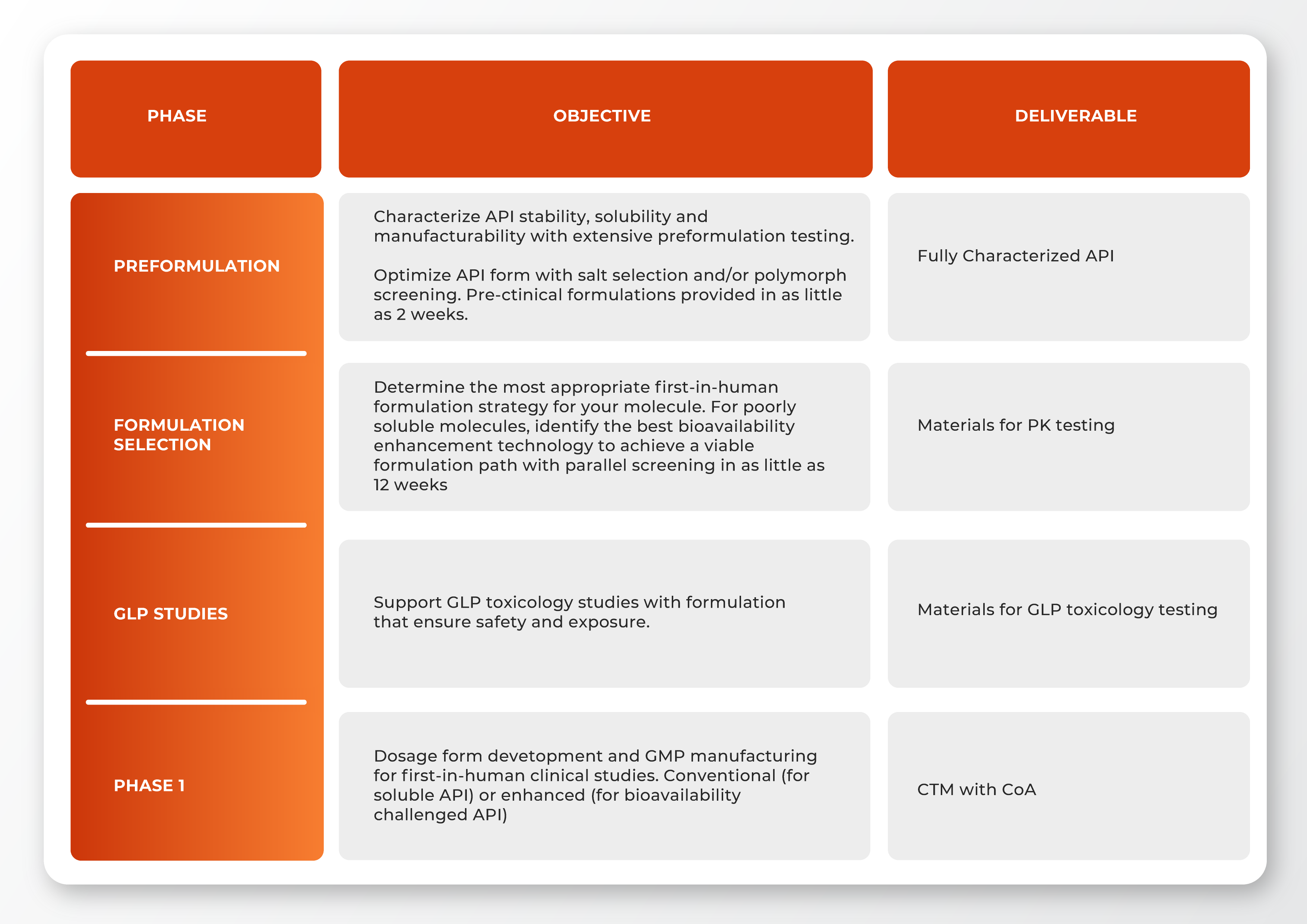
Navigating Early-Phase Challenges
The initial steps of drug development are critical and require a deep understanding of the intricate regulatory environment. At Renejix, we recognize these unique hurdles. Our approach is tailored to effectively navigate this complex landscape, ensuring the meticulous design, development, and collection of high-quality data essential for an Investigational New Drug (IND) submission. Key aspects such as material characterization, solubility analysis, product stability, and safety are handled with the utmost precision and expertise.

Preclinical Studies
In Vitro Studies (Cell Cultures):
- Researchers conduct experiments using cell cultures to assess how the drug interacts with specific cell types. These studies provide insights into the drug’s mechanism of action, potential targets, and initial safety assessments.
- In vitro studies also help identify any adverse effects on cellular structures and functions.
In Vitro Studies (Organoids):
Organoids, three-dimensional cell cultures engineered to mimic real organs, are a cutting-edge advancement in preclinical in vitro research. These structures replicate the complexity of human tissues, making them more physiologically relevant than traditional 2D cell cultures.
Advantages:
- Physiological Relevance: Organoids closely resemble the cell types, composition, and function of target tissues, allowing for more accurate drug testing.
- Disease Modeling: They provide a platform for studying disease mechanisms and drug responses in a controlled in vitro setting.
- Personalized Medicine: Organoids can be derived from patient-specific cells, enabling personalized drug testing.
- Toxicology Testing: Organoids are particularly useful for assessing drug safety. Researchers can observe direct effects on human-like tissue structures without relying solely on animal models.
In Vivo Studies (Animal Models):
- Animal models play a crucial role in preclinical research. Scientists use them to simulate the drug’s effects within a living organism.
- These studies evaluate the drug’s pharmacokinetics (ADME) and its impact on various organs and systems.
- Researchers observe factors such as absorption, distribution, metabolism, and excretion. For instance:
- How quickly does the drug reach the bloodstream after administration?
- How is it processed by the liver?
- What is its half-life within the body?
The Role of ADME in Preclinical Research
Absorption:
- Understanding how the drug is absorbed into the bloodstream is critical. Factors affecting absorption include:
- Route of administration (oral, intravenous, etc.)
- Bioavailability (the fraction of the administered dose that reaches systemic circulation)
- Interaction with food or other drugs
Distribution:
- Researchers study how the drug is distributed throughout the body. Key considerations include:
- Tissue penetration (does it reach target tissues?)
- Binding to plasma proteins
- Blood-brain barrier penetration (relevant for central nervous system drugs)
Metabolism:
- Metabolism occurs primarily in the liver. Researchers investigate:
- Enzymatic breakdown of the drug
- Formation of metabolites (active or inactive)
- Potential drug interactions (e.g., inhibition or induction of liver enzymes)
Excretion:
- Elimination pathways (urine, feces, bile) are assessed.
- Renal excretion is particularly important. Researchers examine:
- Clearance rates
- Renal toxicity
Preclinical Toxicology Testing
Acute Toxicity Studies:
- Short-term studies to assess immediate adverse effects.
- Determine the maximum tolerated dose (MTD) without severe toxicity.
Chronic Toxicity Studies:
- Longer-term studies (weeks to months) to evaluate cumulative effects.
- Observe organ-specific toxicity and potential carcinogenicity.
Specialized Toxicity Assessments:
- Carcinogenicity studies: Identify cancer risks.
- Mutagenicity studies: Evaluate genetic damage potential.
- Reproductive toxicity studies: Assess effects on fertility and fetal development.
Formulation and Stability: Preparing for the Future
Formulation Optimization:
- During the preclinical stage our researchers work on dosage forms (tablets, capsules, injections) that ensure consistent drug delivery.
- Stability studies assess the drug’s shelf life under various conditions (temperature, humidity, light).
Scalability:
- If the drug progresses to clinical trials, it must be scalable for large-scale production.
- Formulation adjustments may be necessary to meet manufacturing requirements.
Preclinical Success Rates: A Measure of Progress
- Preclinical success ensures that only the most promising drugs advance to human trials.
- By filtering out less viable candidates early, our preclinical development program minimizes risks to future clinical trial participants.
Benefits
Preclinical Development: Renejix’s preclinical solutions are designed to overcome the limitations of fixed dose strengths that often hinder phase 1 clinical studies. Our approach allows for flexible dosing adjustments based on preclinical feedback, ensuring a smoother transition to clinical trials.
Phase 1 Clinical Manufacturing: We provide state-of-the-art clinical manufacturing services tailored for phase 1 studies, enabling the production of doses that can be dynamically adjusted in response to real-time clinical data.
Preclinical Formulation Development: Our formulation development is not just cutting-edge but also preclinically informed, allowing for rapid modifications as your drug progresses through preclinical stages and into phase 1 trials.
Integrated Regulatory Support: Renejix offers comprehensive regulatory support that spans both preclinical and phase 1 phases, streamlining the path to First-in-Human studies and beyond.
On-Demand Manufacturing: The power of our on-demand manufacturing lies in its ability to facilitate phase 1 clinical studies by significantly reducing stability requirements, costs, time, and the amount of Active Pharmaceutical Ingredients (API) needed.
Bioavailability Enhancement: Our early phase solutions include advanced bioavailability enhancement techniques that are crucial during both preclinical and phase 1 stages to ensure optimal drug absorption and efficacy.
Preformulation Services: Renejix’s preformulation services are a cornerstone of our early phase solutions, providing the necessary groundwork for successful preclinical and phase 1 development.
IND Enabling Studies: We conduct Investigational New Drug (IND) enabling studies that are essential for both preclinical and phase 1 phases, ensuring that your molecule meets all regulatory requirements before clinical trials begin.
CRO Network Integration: Our integrated network of Clinical Research Organizations (CROs) is adept at navigating the complex landscapes of preclinical and phase 1 studies, expediting your molecule’s development.
Tailored Early Phase Solutions: Renejix’s scientific team offers highly tailored solutions that are informed by both preclinical and phase 1 insights, guiding your molecule along the most optimal development pathway.
Scalable Formulations: Our formulations are not only highly scalable but also designed to facilitate rapid and seamless adjustments during both preclinical and phase 1 stages at the clinical site.
Cost and Time Efficiency: Renejix’s approach to drug development prioritizes large cost and time savings, a benefit that extends from preclinical research through to phase 1 clinical trials.
Data-Driven Clinical Insights: We empower data-driven decision-making by providing real-time clinical insights that inform both preclinical and phase 1 development strategies.
Regulatory Filing Strategies: Our integrated approach improves customers’ regulatory filing strategies, offering benefits throughout preclinical and phase 1 processes.
Optimized Treatment Performance: Renejix’s solutions are designed to generate a faster onset of action, reduce API dose, minimize side effects, and improve overall treatment performance from preclinical stages to phase 1 trials.
Commercially Successful Dosage Forms: We reduce clinical development time through dosage forms that have a strong track record of commercial success, relevant to both preclinical and phase 1 phases.
Handling of Difficult APIs: Renejix specializes in the handling and manufacturing of difficult APIs, providing expert services that are crucial during both preclinical and phase 1 stages.
Stages
Formulation Development:
- Preclinical Formulation Techniques: Utilizing a range of technologies such as spray drying, hot melt extrusion, or lipid-based methods, we design phase-appropriate intermediate products that cater to both preclinical and phase 1 requirements.
- Flexible Formulation Design Space: Our mapped formulation design space is critical in preclinical stages, allowing for the selection of the optimal formulation and dose, adaptable for phase 1 clinical trials.
Product Stability:
- Preclinical Stability Evaluation: We assess in-use stability within the intended format, such as blends in capsules or bottles, ensuring robust product integrity from preclinical to phase 1 dose ranges.
GMP Manufacturing:
- Phase 1 GMP Standards: Our production of scalable, single batches for clinical tests adheres to Good Manufacturing Practice (GMP) standards, crucial for phase 1 clinical trials.
- Phase 1 On-Demand Manufacturing: We efficiently fill bulk intermediate products into prepared capsules or bottles on-demand at CROs’ Phase 1 Clinics, streamlining the phase 1 study process.
Iterative Dosing:
- Preclinical to Phase 1 Dose Iteration: Establishing calculated dose increments based on emerging clinical data is of utmost importance, bridging preclinical findings with phase 1 clinical adjustments.
In-Depth Review of Clinical Data:
- Phase 1 Data Review: In-depth analysis of clinical data enables prudent dose adjustments, informed by both preclinical and phase 1 insights.
- Preclinical and Phase 1 Decision Cycles: We allow up to three weeks for iterative cycles between dosings, a practice that benefits both preclinical and phase 1 stages.
Regulatory Support:
- Preclinical to Phase 1 Regulatory Pathway: We expedite the path to the clinic by leveraging data from non-clinical batches and preparing a comprehensive CMC section for regulatory submissions, justifying dose ranges that facilitate seamless adjustments during phase 1 clinical trials.
Bioavailability Studies:
- Preclinical Bioavailability Optimization: Our preclinical studies focus on optimizing bioavailability, which is a critical factor when advancing to phase 1 clinical trials.
API Optimization:
- Preclinical API Scaling: Our preclinical work includes optimizing the API for scalability, ensuring a smooth transition to phase 1 manufacturing processes.
Clinical Supply Chain Management:
- Phase 1 Supply Chain Efficiency: We manage the clinical supply chain to ensure efficient and timely delivery of materials for phase 1 studies.
Safety and Efficacy Metrics:
- Preclinical Safety to Phase 1 Efficacy: We establish safety and efficacy metrics in preclinical stages that guide phase 1 clinical evaluations.
Facilities
Our facilities are equipped to handle the transition from preclinical to phase 1 manufacturing, ensuring a smooth progression in the drug development pipeline. We ensure compliance with all regulatory standards, providing capabilities that span from non-sterile oral dosage form development in preclinical studies to analytical support and small-scale commercial manufacturing for phase 1 trials. We offer preclinical, clinical, and commercial commercial manufacturing for non-sterile oral and topical dosage forms.
Preclinical Research and Development: Our two plants are not only dedicated to turnkey oral dosage development but also excel in preclinical research and development, ensuring that our formulations meet the stringent requirements of phase 1 clinical trials.
Preclinical Analytical Support: Our analytical support extends from preclinical development, providing a solid foundation for phase 1 clinical trials.
Phase 1 Pilot Line: The pilot line is crucial for scaling up from preclinical to phase 1, providing a seamless transition for oral dosage forms under development.
Phase 1 Manufacturing Flexibility: We offer small-scale commercial manufacturing for non-sterile oral dosage forms, tailored to the needs of phase 1 clinical studies.
Controlled Drug Capabilities: With commercial lines that feature controlled drug capabilities, we maintain a robust pipeline from preclinical stages to phase 1 and beyond, all under FDA audit.
Quality and Regulatory Compliance: Our four oral solids facilities boast a strong track record for both quality and regulatory compliance, from preclinical research through phase 1 trials and commercial production.
Solvent-Based Processing: We use Category I-IV solvent-based processing techniques that are optimized for both preclinical and phase 1 development phases.
Extrusion and Spheronization: These techniques are integral to our preclinical development process, setting the stage for successful phase 1 clinical trials.
High Shear Granulation: Our commercial high shear granulation capabilities are designed to meet the rigorous demands of phase 1 clinical manufacturing.
Blending Blending is a critical step in both preclinical and phase 1 processes, ensuring uniformity and consistency in oral dosage forms.
Tablet Compression: We have advanced tablet compression capabilities that serve both preclinical studies and phase 1 clinical trials.
Hardshell Capsule Filling: Our hardshell capsule filling processes are fine-tuned during preclinical development to ensure precision and reliability in phase 1 trials.
Pan-Coating and Fluid Bed Techniques: These are employed to enhance product stability and efficacy from preclinical stages to phase 1 clinical trials.
Dry Granulation: Optimized for preclinical development, our dry granulation process ensures granules meet phase 1 clinical trial specifications and maintains quality during the transition from preclinical to phase 1 production.
Non-sterile Oral Dosage Form Development: Specializing in non-sterile oral dosage forms during the preclinical phase, we optimize these forms based on clinical feedback for phase 1 clinical evaluation.
Analytical Support for Oral Dosage Form Development: Providing robust analytical support during the preclinical phase, we ensure comprehensive data informs phase 1 development decisions and regulatory compliance.
Regulatory Strategy: We develop a comprehensive regulatory strategy that encompasses both preclinical and phase 1 stages, facilitating a streamlined approval process.
Dosage Form Development
Preclinical to Phase 1 Strategy: Our strategy for early human studies involves a partnership to develop a robust formulation plan that evolves from preclinical findings to meet the specific needs of phase 1 human trials. We will guide your from preclinical development through the milestones of phase 1 clinical trials.
cGMP Standards in Manufacturing: Upholding high-quality manufacturing, Renejix meets strict cGMP standards, a commitment that begins in the preclinical phase and extends through phase 1 and beyond.
Accelerating Clinical Trials: By streamlining processes from preclinical stages, we expedite the initiation of phase 1 clinical trials, ensuring faster progress for our partners.
-
Preclinical Studies
-
The Role of ADME in Preclinical Research
-
Preclinical Toxicology Testing
-
Formulation and Stability: Preparing for the Future
-
Preclinical Success Rates: A Measure of Progress
-
Benefits
-
Stages
-
Facilities
-
Dosage Form Development
In Vitro Studies (Cell Cultures):
- Researchers conduct experiments using cell cultures to assess how the drug interacts with specific cell types. These studies provide insights into the drug’s mechanism of action, potential targets, and initial safety assessments.
- In vitro studies also help identify any adverse effects on cellular structures and functions.
In Vitro Studies (Organoids):
Organoids, three-dimensional cell cultures engineered to mimic real organs, are a cutting-edge advancement in preclinical in vitro research. These structures replicate the complexity of human tissues, making them more physiologically relevant than traditional 2D cell cultures.
Advantages:
- Physiological Relevance: Organoids closely resemble the cell types, composition, and function of target tissues, allowing for more accurate drug testing.
- Disease Modeling: They provide a platform for studying disease mechanisms and drug responses in a controlled in vitro setting.
- Personalized Medicine: Organoids can be derived from patient-specific cells, enabling personalized drug testing.
- Toxicology Testing: Organoids are particularly useful for assessing drug safety. Researchers can observe direct effects on human-like tissue structures without relying solely on animal models.
In Vivo Studies (Animal Models):
- Animal models play a crucial role in preclinical research. Scientists use them to simulate the drug’s effects within a living organism.
- These studies evaluate the drug’s pharmacokinetics (ADME) and its impact on various organs and systems.
- Researchers observe factors such as absorption, distribution, metabolism, and excretion. For instance:
- How quickly does the drug reach the bloodstream after administration?
- How is it processed by the liver?
- What is its half-life within the body?
Absorption:
- Understanding how the drug is absorbed into the bloodstream is critical. Factors affecting absorption include:
- Route of administration (oral, intravenous, etc.)
- Bioavailability (the fraction of the administered dose that reaches systemic circulation)
- Interaction with food or other drugs
Distribution:
- Researchers study how the drug is distributed throughout the body. Key considerations include:
- Tissue penetration (does it reach target tissues?)
- Binding to plasma proteins
- Blood-brain barrier penetration (relevant for central nervous system drugs)
Metabolism:
- Metabolism occurs primarily in the liver. Researchers investigate:
- Enzymatic breakdown of the drug
- Formation of metabolites (active or inactive)
- Potential drug interactions (e.g., inhibition or induction of liver enzymes)
Excretion:
- Elimination pathways (urine, feces, bile) are assessed.
- Renal excretion is particularly important. Researchers examine:
- Clearance rates
- Renal toxicity
Acute Toxicity Studies:
- Short-term studies to assess immediate adverse effects.
- Determine the maximum tolerated dose (MTD) without severe toxicity.
Chronic Toxicity Studies:
- Longer-term studies (weeks to months) to evaluate cumulative effects.
- Observe organ-specific toxicity and potential carcinogenicity.
Specialized Toxicity Assessments:
- Carcinogenicity studies: Identify cancer risks.
- Mutagenicity studies: Evaluate genetic damage potential.
- Reproductive toxicity studies: Assess effects on fertility and fetal development.
Formulation Optimization:
- During the preclinical stage our researchers work on dosage forms (tablets, capsules, injections) that ensure consistent drug delivery.
- Stability studies assess the drug’s shelf life under various conditions (temperature, humidity, light).
Scalability:
- If the drug progresses to clinical trials, it must be scalable for large-scale production.
- Formulation adjustments may be necessary to meet manufacturing requirements.
- Preclinical success ensures that only the most promising drugs advance to human trials.
- By filtering out less viable candidates early, our preclinical development program minimizes risks to future clinical trial participants.
Preclinical Development: Renejix’s preclinical solutions are designed to overcome the limitations of fixed dose strengths that often hinder phase 1 clinical studies. Our approach allows for flexible dosing adjustments based on preclinical feedback, ensuring a smoother transition to clinical trials.
Phase 1 Clinical Manufacturing: We provide state-of-the-art clinical manufacturing services tailored for phase 1 studies, enabling the production of doses that can be dynamically adjusted in response to real-time clinical data.
Preclinical Formulation Development: Our formulation development is not just cutting-edge but also preclinically informed, allowing for rapid modifications as your drug progresses through preclinical stages and into phase 1 trials.
Integrated Regulatory Support: Renejix offers comprehensive regulatory support that spans both preclinical and phase 1 phases, streamlining the path to First-in-Human studies and beyond.
On-Demand Manufacturing: The power of our on-demand manufacturing lies in its ability to facilitate phase 1 clinical studies by significantly reducing stability requirements, costs, time, and the amount of Active Pharmaceutical Ingredients (API) needed.
Bioavailability Enhancement: Our early phase solutions include advanced bioavailability enhancement techniques that are crucial during both preclinical and phase 1 stages to ensure optimal drug absorption and efficacy.
Preformulation Services: Renejix’s preformulation services are a cornerstone of our early phase solutions, providing the necessary groundwork for successful preclinical and phase 1 development.
IND Enabling Studies: We conduct Investigational New Drug (IND) enabling studies that are essential for both preclinical and phase 1 phases, ensuring that your molecule meets all regulatory requirements before clinical trials begin.
CRO Network Integration: Our integrated network of Clinical Research Organizations (CROs) is adept at navigating the complex landscapes of preclinical and phase 1 studies, expediting your molecule’s development.
Tailored Early Phase Solutions: Renejix’s scientific team offers highly tailored solutions that are informed by both preclinical and phase 1 insights, guiding your molecule along the most optimal development pathway.
Scalable Formulations: Our formulations are not only highly scalable but also designed to facilitate rapid and seamless adjustments during both preclinical and phase 1 stages at the clinical site.
Cost and Time Efficiency: Renejix’s approach to drug development prioritizes large cost and time savings, a benefit that extends from preclinical research through to phase 1 clinical trials.
Data-Driven Clinical Insights: We empower data-driven decision-making by providing real-time clinical insights that inform both preclinical and phase 1 development strategies.
Regulatory Filing Strategies: Our integrated approach improves customers’ regulatory filing strategies, offering benefits throughout preclinical and phase 1 processes.
Optimized Treatment Performance: Renejix’s solutions are designed to generate a faster onset of action, reduce API dose, minimize side effects, and improve overall treatment performance from preclinical stages to phase 1 trials.
Commercially Successful Dosage Forms: We reduce clinical development time through dosage forms that have a strong track record of commercial success, relevant to both preclinical and phase 1 phases.
Handling of Difficult APIs: Renejix specializes in the handling and manufacturing of difficult APIs, providing expert services that are crucial during both preclinical and phase 1 stages.
Formulation Development:
- Preclinical Formulation Techniques: Utilizing a range of technologies such as spray drying, hot melt extrusion, or lipid-based methods, we design phase-appropriate intermediate products that cater to both preclinical and phase 1 requirements.
- Flexible Formulation Design Space: Our mapped formulation design space is critical in preclinical stages, allowing for the selection of the optimal formulation and dose, adaptable for phase 1 clinical trials.
Product Stability:
- Preclinical Stability Evaluation: We assess in-use stability within the intended format, such as blends in capsules or bottles, ensuring robust product integrity from preclinical to phase 1 dose ranges.
GMP Manufacturing:
- Phase 1 GMP Standards: Our production of scalable, single batches for clinical tests adheres to Good Manufacturing Practice (GMP) standards, crucial for phase 1 clinical trials.
- Phase 1 On-Demand Manufacturing: We efficiently fill bulk intermediate products into prepared capsules or bottles on-demand at CROs’ Phase 1 Clinics, streamlining the phase 1 study process.
Iterative Dosing:
- Preclinical to Phase 1 Dose Iteration: Establishing calculated dose increments based on emerging clinical data is of utmost importance, bridging preclinical findings with phase 1 clinical adjustments.
In-Depth Review of Clinical Data:
- Phase 1 Data Review: In-depth analysis of clinical data enables prudent dose adjustments, informed by both preclinical and phase 1 insights.
- Preclinical and Phase 1 Decision Cycles: We allow up to three weeks for iterative cycles between dosings, a practice that benefits both preclinical and phase 1 stages.
Regulatory Support:
- Preclinical to Phase 1 Regulatory Pathway: We expedite the path to the clinic by leveraging data from non-clinical batches and preparing a comprehensive CMC section for regulatory submissions, justifying dose ranges that facilitate seamless adjustments during phase 1 clinical trials.
Bioavailability Studies:
- Preclinical Bioavailability Optimization: Our preclinical studies focus on optimizing bioavailability, which is a critical factor when advancing to phase 1 clinical trials.
API Optimization:
- Preclinical API Scaling: Our preclinical work includes optimizing the API for scalability, ensuring a smooth transition to phase 1 manufacturing processes.
Clinical Supply Chain Management:
- Phase 1 Supply Chain Efficiency: We manage the clinical supply chain to ensure efficient and timely delivery of materials for phase 1 studies.
Safety and Efficacy Metrics:
- Preclinical Safety to Phase 1 Efficacy: We establish safety and efficacy metrics in preclinical stages that guide phase 1 clinical evaluations.
Our facilities are equipped to handle the transition from preclinical to phase 1 manufacturing, ensuring a smooth progression in the drug development pipeline. We ensure compliance with all regulatory standards, providing capabilities that span from non-sterile oral dosage form development in preclinical studies to analytical support and small-scale commercial manufacturing for phase 1 trials. We offer preclinical, clinical, and commercial commercial manufacturing for non-sterile oral and topical dosage forms.
Preclinical Research and Development: Our two plants are not only dedicated to turnkey oral dosage development but also excel in preclinical research and development, ensuring that our formulations meet the stringent requirements of phase 1 clinical trials.
Preclinical Analytical Support: Our analytical support extends from preclinical development, providing a solid foundation for phase 1 clinical trials.
Phase 1 Pilot Line: The pilot line is crucial for scaling up from preclinical to phase 1, providing a seamless transition for oral dosage forms under development.
Phase 1 Manufacturing Flexibility: We offer small-scale commercial manufacturing for non-sterile oral dosage forms, tailored to the needs of phase 1 clinical studies.
Controlled Drug Capabilities: With commercial lines that feature controlled drug capabilities, we maintain a robust pipeline from preclinical stages to phase 1 and beyond, all under FDA audit.
Quality and Regulatory Compliance: Our four oral solids facilities boast a strong track record for both quality and regulatory compliance, from preclinical research through phase 1 trials and commercial production.
Solvent-Based Processing: We use Category I-IV solvent-based processing techniques that are optimized for both preclinical and phase 1 development phases.
Extrusion and Spheronization: These techniques are integral to our preclinical development process, setting the stage for successful phase 1 clinical trials.
High Shear Granulation: Our commercial high shear granulation capabilities are designed to meet the rigorous demands of phase 1 clinical manufacturing.
Blending Blending is a critical step in both preclinical and phase 1 processes, ensuring uniformity and consistency in oral dosage forms.
Tablet Compression: We have advanced tablet compression capabilities that serve both preclinical studies and phase 1 clinical trials.
Hardshell Capsule Filling: Our hardshell capsule filling processes are fine-tuned during preclinical development to ensure precision and reliability in phase 1 trials.
Pan-Coating and Fluid Bed Techniques: These are employed to enhance product stability and efficacy from preclinical stages to phase 1 clinical trials.
Dry Granulation: Optimized for preclinical development, our dry granulation process ensures granules meet phase 1 clinical trial specifications and maintains quality during the transition from preclinical to phase 1 production.
Non-sterile Oral Dosage Form Development: Specializing in non-sterile oral dosage forms during the preclinical phase, we optimize these forms based on clinical feedback for phase 1 clinical evaluation.
Analytical Support for Oral Dosage Form Development: Providing robust analytical support during the preclinical phase, we ensure comprehensive data informs phase 1 development decisions and regulatory compliance.
Regulatory Strategy: We develop a comprehensive regulatory strategy that encompasses both preclinical and phase 1 stages, facilitating a streamlined approval process.
Preclinical to Phase 1 Strategy: Our strategy for early human studies involves a partnership to develop a robust formulation plan that evolves from preclinical findings to meet the specific needs of phase 1 human trials. We will guide your from preclinical development through the milestones of phase 1 clinical trials.
cGMP Standards in Manufacturing: Upholding high-quality manufacturing, Renejix meets strict cGMP standards, a commitment that begins in the preclinical phase and extends through phase 1 and beyond.
Accelerating Clinical Trials: By streamlining processes from preclinical stages, we expedite the initiation of phase 1 clinical trials, ensuring faster progress for our partners.

Our Services
FAQs
Here are some frequently asked questions about Early Phase
During Phase 1 clinical trials, we play a critical role by:
- Conducting Preformulation Studies: These are essential to understand the drug’s physical and chemical properties, which inform the formulation strategy.
- Developing Formulations: Creating stable and effective dosage forms that are crucial for the success of early-stage clinical trials.
- Establishing Analytical Methods: Ensuring the drug product meets stringent quality standards, which is vital for regulatory approval.
- Manufacturing Clinical Trial Materials: Producing small-scale batches for Phase 1 trials, adhering to Good Manufacturing Practices (GMP) to ensure safety and quality.
- Providing Regulatory Support: Offering expertise to navigate the complex regulatory requirements specific to Phase 1 trials, including documentation and compliance strategies.
Our expertise in formulation development is multifaceted, encompassing:
- Advanced Drug Delivery Systems: Tailoring the method of drug administration to enhance patient compliance and drug effectiveness.
- Solving Solubility and Stability Issues: Utilizing cutting-edge technologies and innovative approaches to overcome common challenges in drug formulation.
- Enhancing Bioavailability: Implementing strategies to ensure the drug is absorbed effectively in the body, which is crucial for therapeutic efficacy.
Ensuring the quality of clinical trial materials is a top priority for us, achieved through:
- Robust Quality Management Systems: These systems are designed to uphold the highest standards of quality throughout the manufacturing process.
- GMP Compliance: Adhering to GMP regulations is non-negotiable, ensuring that products are consistently produced and controlled according to quality standards.
- Comprehensive Analytical Testing: Rigorous testing protocols are in place to confirm the identity, purity, potency, and stability of the clinical trial materials.
Scaling up from Phase 1 to subsequent trial phases is a complex process that we are well-equipped to handle, thanks to:
- Scalable Processes: Designed to be adaptable, these processes can transition from small-scale production for Phase 1 to larger scales for subsequent phases.
- Tech Transfer Capabilities: The ability to transfer technology efficiently is crucial for scaling up production without compromising quality or timelines.
- Supply Chain Management: A robust supply chain is essential to ensure the availability of high-quality raw materials and components as production scales up.
We comprehensive regulatory support including:
- Documentation Preparation: Investigational New Drug (IND) applications and other regulatory documents that are critical for initiating clinical trials.
- Strategic Regulatory Guidance: Developing and implementing regulatory strategies that align with the guidelines of the FDA and other regulatory bodies, ensuring a smooth and compliant trial process.
- Compliance Consultation: Providing expert advice and consultation to ensure all regulatory requirements are met, which is essential for the successful progression of clinical trials.
Project management for Phase 1 trials involves:
- Dedicated Project Teams: These teams are committed to the success of your project, with a focus on meeting your specific needs.
- Effective Communication: Keeping lines of communication open and transparent to ensure that all stakeholders are aligned and informed.
- Timeline Management: Strict adherence to project timelines is essential to meet the critical milestones of Phase 1 trials.
A CDMO’s manufacturing process is tailored to meet the unique requirements of Phase 1 trials through:
- Customized Manufacturing Processes: Tailoring manufacturing processes to fit the specific needs of each trial, ensuring the production of trial materials that meet all therapeutic and regulatory requirements.
- Rapid Production Capabilities: The ability to produce trial materials quickly and efficiently is essential to meet the often tight schedules of Phase 1 trials.
- High Precision: Ensuring that each batch of trial materials is produced with the utmost accuracy and consistency.
- Specialized Equipment and Facilities: To accommodate the unique requirements of complex formulations, including those that require containment.
- Expert Personnel: Teams of highly skilled professionals with extensive experience in addressing the challenges of complex drug development.
- Innovative Techniques and Technologies: Employing the latest techniques and technologies to effectively manage the unique properties of complex drug molecules, ensuring their stability, efficacy, and safety.
The analytical capabilities of a CDMO are critical for:
- Method Validation: Ensuring that the analytical methods used are reliable and reproducible.
- Stability Studies: Conducting thorough stability studies to assess how the drug maintains its integrity over time, which is crucial for determining appropriate storage conditions and shelf life.
- Release Testing: Performing meticulous release testing to verify that each batch of the drug product meets all specified criteria before it is released for use in clinical trials.
Our contribution to the success of your Phase 1 clinical trials is multifaceted, including:
- Proactive Risk Management: Identifying potential issues early in the development process and implementing strategies to mitigate these risks.
- Resource Optimization: Streamlining development processes to maximize resource utilization and minimize costs, without compromising quality or safety.
- Strategic Expertise: Leveraging extensive industry experience to provide strategic insights and best practices that can significantly enhance the drug development process.





















