Oral suspensions and solutions are a cornerstone of pharmaceutical formulations, offering a versatile and often more palatable alternative to solid dosage forms. Scaling up liquid formulations can present a number of challenges related to mixing, solubility, viscosity and filtration. With complex solutions and suspension, these issues can become even more magnified as the liquid formulation transitions from feasibility to commercialization. We specialize in oral solutions and suspensions, even for pediatric medicines.
Our experts are ready to tackle the unique challenges of making and packaging your liquid product and get your product to market smoothly and confidently.

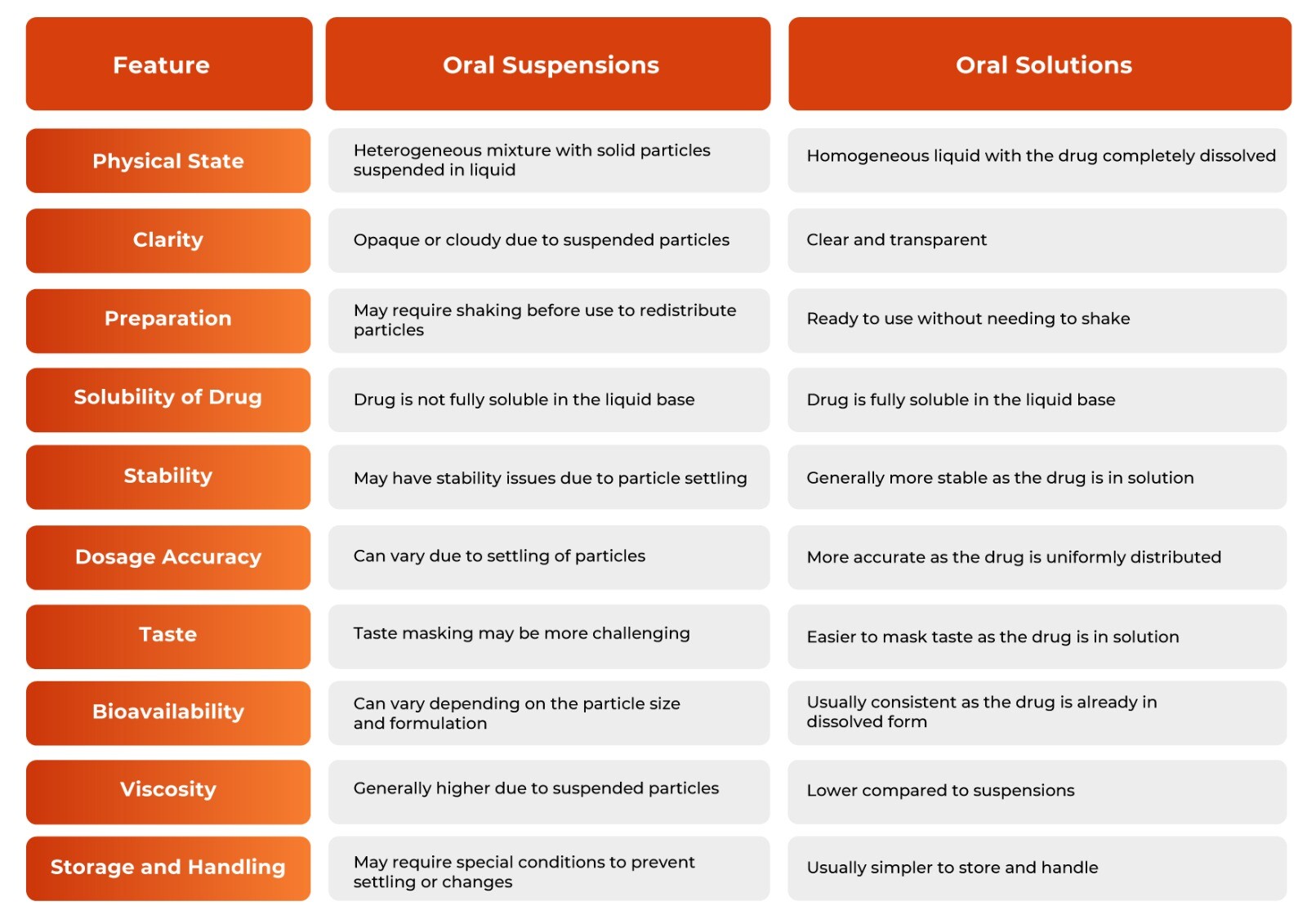
Oral Suspensions vs Oral Solutions
Oral Suspension Manufacturing Capabilities
- Pilot, Clinical Trial Material, and Commercial-Scale Manufacturing: We offer comprehensive services for oral solutions, from initial pilot batches to full-scale commercial manufacturing, adhering to the highest industry standards.
- Segregated Manufacturing Suites: Our facilities feature dedicated suites for oral suspensions, preventing cross-contamination and ensuring product integrity.
- Liquid cGMP Batch Sizes from 10 L to 4,400 L: We can accommodate a wide range of batch sizes for oral solutions, ensuring flexibility and scalability for our clients’ needs.
- Suspension cGMP Batch Sizes from 10 L to 4,400 L: Similar to our liquid capabilities, we can produce oral suspensions in various batch sizes, catering to both small-scale and large-scale production requirements.
- Compartmentalized, Controlled Environment and XP Liquid Compounding: Our state-of-the-art compounding areas are designed for the precise creation of oral solutions, maintaining strict environmental control throughout the process.
- Controlled Substances (CI – CV): We are licensed to handle a variety of controlled substances, producing both oral suspensions and oral solutions that meet regulatory compliance.
- Extensive Range of Vessel, Filtration, and Mixing Options: Our versatile equipment allows for the optimal production of oral solutions, with a focus on quality and consistency.
- Vacuum-Rated, Steam-Heated and Water-Cooled Jacketed Vessels: These specialized vessels are crucial for manufacturing oral suspensions that require precise temperature control during production.
- HEPA Air Filtration: High-Efficiency Particulate Air (HEPA) filters ensure a contaminant-free environment for the production of both oral solutions and oral suspensions.
- Facility Lighting Options for Light-Sensitive Materials: We provide specialized lighting for the safe handling and manufacturing of light-sensitive oral solutions and oral suspensions.
- Advanced Analytical Testing: Every batch of oral suspension and oral solution undergoes rigorous analytical testing to ensure compliance with all specifications and regulations.
- Custom Formulation Development: Our team excels in developing custom formulations for oral solutions, tailored to meet unique therapeutic and market demands.
- Packaging and Labeling Solutions: We offer a variety of packaging options for oral suspensions, designed to preserve product stability and extend shelf life.
- Regulatory Support: Our regulatory experts provide comprehensive support for oral solutions, assisting with documentation and submission for regulatory approval.
Liquid Capabilities
- Oral Liquids: Our facilities are equipped to produce a variety of oral solutions, ranging from simple formulations to complex multicomponent liquids, ensuring versatility and precision in liquid dosage forms.
- Taste-Masking: We specialize in advanced taste-masking techniques for oral suspensions, enhancing patient compliance by improving the palatability of bitter or otherwise unpleasant-tasting medications.
- Aqueous and Solvent-Based Systems and Premixes: Our expertise extends to both water-based and solvent-based oral solutions, providing the foundation for a wide array of therapeutic products.
- High-Pressure and High-Shear Homogenization: Utilizing cutting-edge homogenization technologies, we can achieve uniform particle distribution in oral suspensions, crucial for dose consistency and stability.
- Product Heating and Cooling: Our state-of-the-art equipment allows for precise temperature control during the manufacturing of oral solutions, ensuring optimal conditions for heat-sensitive ingredients.
- Microencapsulation: This technology allows us to create oral suspensions with microencapsulated active ingredients, offering controlled release and targeted delivery.
- Sterile Filtration: We employ sterile filtration processes to ensure the microbiological safety of oral solutions, meeting stringent regulatory standards.
- Stability Testing: Our comprehensive stability testing protocols for oral suspensions ensure that products maintain their efficacy and safety throughout their shelf life.
- Regulatory Compliance: We navigate the complex regulatory landscape to ensure that all oral solutions meet the necessary guidelines and approvals for market entry.
Packaging Capabilities
- Glass or Plastic Bottles: We offer packaging solutions for oral suspensions in both glass and plastic bottles, catering to the specific needs of stability and compatibility.
- Sizes from 10 mL to 4 L: Our packaging lines can fill a variety of sizes, from small 10 mL bottles perfect for pediatric oral solutions, up to large 4 L containers for bulk supply.
- Child-Resistant Closures: Safety is a priority, and our child-resistant closures for oral suspensions help prevent accidental ingestion by children.
- Tamper-Evident Seals: We ensure the integrity of our oral solutions with tamper-evident seals, providing peace of mind for both providers and patients.
- Labeling Accuracy: High-precision labeling systems are in place to ensure that every bottle of oral suspension is accurately labeled with all necessary information.
- Serialization and Aggregation: Advanced serialization and aggregation techniques are used for oral solutions to meet global regulatory requirements and ensure supply chain security.
Why Oral Suspensions & Oral Liquids?
- Enhanced Bioavailability: Both oral suspensions and oral solutions are designed to maximize the bioavailability of medications. This is particularly advantageous for drugs that are poorly soluble in their solid form, as the active ingredients in oral solutions are already dissolved, allowing for quicker absorption and more immediate therapeutic effects.
- Dosing Flexibility: The liquid nature of oral suspensions provides unmatched dosing flexibility, which is essential for tailoring dosages to individual patient requirements, particularly in pediatric and geriatric care where precision is paramount.
- Ease of Administration: Oral solutions are often more user-friendly than their solid counterparts, especially for patients who struggle with swallowing pills. This ease of administration is a significant advantage in ensuring that patients of all ages can take their medications without difficulty.
- Improved Stability: Some medications are inherently more stable as liquids, and oral suspensions can offer enhanced stability for compounds that are prone to degradation when in solid form.
- Controlled Release: By formulating active pharmaceutical ingredients (APIs) into oral suspensions, it’s possible to engineer a controlled release mechanism. This allows the medication to be released slowly over time, providing sustained therapeutic effects and improving patient outcomes.
- Taste Masking: An essential feature of oral suspensions is the ability to mask the taste of certain APIs, which can be bitter or unpleasant. This improves the palatability of medications, leading to better adherence and patient compliance.
- Patient Compliance: The combination of ease of use, palatable taste, and the option to take the medication without water all contribute to higher patient compliance rates for both oral suspensions and oral solutions.
- Transportation and Storage: Oral solutions and oral suspensions are generally easy to transport and store, which is a significant advantage over some biologics or other temperature-sensitive medications that may require special storage conditions.
- Customization Potential: There is a high degree of customization available with oral suspensions and oral solutions, allowing for the addition of colors, flavors, and sweeteners to meet the preferences of different patient groups, enhancing the appeal and acceptance of these medications across various demographics.
Related Services





FAQs
Here are some frequently asked questions about Oral Suspensions & Oral Solutions
Oral suspensions and solutions are two popular liquid dosage forms used for the administration of medications. An oral solution is a clear, homogeneous liquid containing one or more dissolved substances, typically intended for ingestion. Oral suspension, on the other hand, consists of solid particles dispersed throughout a liquid in which they are not soluble, requiring shaking before administration to ensure dose uniformity. Both forms are designed to improve ease of use, enhance patient compliance, and provide an effective means of dose adjustment.
Oral suspensions and solutions are particularly beneficial for patients who have difficulty swallowing tablets or capsules, such as children and elderly individuals. They allow for flexible dosing, can be flavored to improve palatability, and can offer faster onset of action compared to solid dosage forms due to their liquid nature and the consequent rapid absorption. They are also suitable for active ingredients that are unstable or insoluble in solid form.
Stability is ensured through rigorous formulation development, which includes the selection of appropriate stabilizers, preservatives, and pH adjusters. Stability testing under various environmental conditions (temperature, humidity, and light) is conducted to determine the shelf life. Additionally, packaging is carefully chosen to protect the product from environmental factors and contamination.
Formulating oral suspensions and solutions involves several key considerations, including solubility enhancement, taste masking, viscosity adjustment, and preservative efficacy. The formulation must ensure physical and chemical stability, acceptable palatability, and compliance with regulatory requirements. The choice of excipients and the manufacturing process must also be optimized to ensure product quality and consistency.
Yes, oral suspensions and solutions can be customized for specific patient populations, such as pediatric, geriatric, or patients with specific dietary restrictions. This customization can include flavoring, sweetening, viscosity adjustments, and formulation free from allergens or certain additives, providing a tailored medication solution that enhances patient compliance and treatment outcomes.
Quality control is integral to the manufacturing process and includes both in-process controls and finished product testing. Parameters such as pH, viscosity, particle size distribution (for suspensions), microbial limits, and the concentration of active ingredients are rigorously tested. Compliance with Good Manufacturing Practice (GMP) standards ensures the consistency, safety, and efficacy of every batch produced.
Packaging options include bottles, sachets, and single-dose containers, made from materials that protect the product from light, air, and microbial contamination. Child-resistant closures and tamper-evident seals are standard features. The packaging material and design are selected based on the product's stability requirements and intended use.
Flavors and sweeteners are carefully selected based on their compatibility with the active ingredients and other excipients, as well as their stability over the product's intended shelf life. Consumer acceptance testing is often conducted to ensure the chosen flavors are well-received by the target patient population, with particular attention to pediatric formulations where palatability is crucial for compliance.
Oral suspensions and solutions must comply with regulatory requirements set forth by health authorities such as the FDA, EMA, and other national agencies. This includes compliance with monographs (if applicable), submission of stability data, adherence to GMP practices, and demonstration of safety and efficacy through appropriate clinical trials. Labeling must also provide comprehensive dosing instructions, storage conditions, and safety information.
Hycon Labs offers end-to-end services for oral suspensions and solutions, including formulation development, analytical method development, stability testing, clinical trial supply manufacturing, scale-up, and commercial manufacturing. With expertise in regulatory compliance and quality assurance, Hycon can streamline the path from concept to market, ensuring the delivery of high-quality, effective medications tailored to client specifications and patient needs.